DO YOU CARE FOR A CHILD, ADOLESCENT OR YOUNG ADULT WITH FRAGILE X?
People with Fragile X syndrome (FXS) often experience behavioural symptoms such as:

Anxiety — particularly social anxiety / avoidance
Irritability

Tantrums
Social unresponsiveness
The behavioural symptoms associated with a Fragile X diagnosis can create daily challenges for your child, your family and you. A clinical trial known as RECONNECT is evaluating an investigational, topical treatment that may help improve Fragile X-related behavioural symptoms.

WHAT ARE THE RECONNECT STUDY QUALIFICATIONS?
Between the ages of 3 and 22
Diagnosed with FXS via genetic testing
Experiencing behavioural symptoms of FXS
In generally good health
HOW IS THE MEDICATION ADMINISTERED?
The dose of study medication depends on the weight of the participant:
≤ 66 lbs (30 kg)

2 PACKETS OF GEL/DAY
- 1 packet approximately every 12 hours
- Total daily dose of 250 mg
>66 lbs (30 kg) to ≤ 110 lbs (50 kg)

4 PACKETS OF GEL/DAY
- 2 packets approximately every 12 hours
- Total daily dose of 500 mg
> 110 lbs (50 kg)

6 PACKETS OF GEL/DAY
- 3 packets approximately every 12 hours
- Total daily dose of 750 mg
Parents or caregivers will be instructed on proper application of the gel to clean, dry, intact skin of the upper arms/shoulders.
WHERE ARE THE RECONNECT CLINICAL TRIAL CENTERS LOCATED?

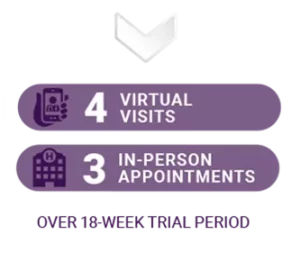
HOW OFTEN WILL MY CHILD NEED TO VISIT THE TRIAL SITE IF ELIGIBLE?
After the initial screening visit to confirm eligibility, participants will have: